With a range of rapid tests based on the GENSPEED µELISA platform, GENSPEED offers a product portfolio with great value for the entire society. The GENSPEED Vitamin D xPOC has been available since September 2024 and is approved in accordance with the In-Vitro Diagnostic Regulation (EU 2017/746)
Vitamin D xPOC - Rapid Quantification with µELISA
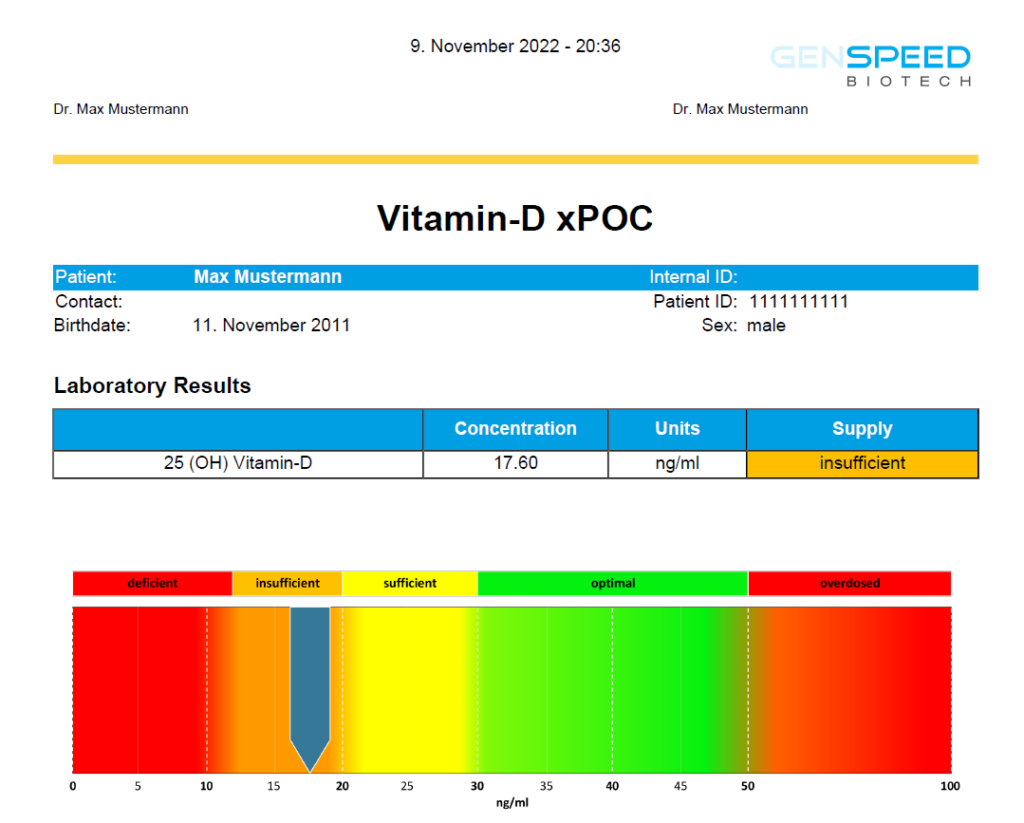
The GENSPEED® Vitamin D xPOC test is an in vitro diagnostic tool for the quantitative detection of 25(OH) Vitamin D3 from human capillary blood samples and venous blood samples. The test can be performed with 50 µl of blood from the fingertip in about 20 minutes, delivering laboratory-quality results.
This is made possible by the patented GENSPEED® micro-ELISA technology. By combining microfluidics with state-of-the-art optical readout technology, the test duration of a standard laboratory ELISA is reduced from 2 – 4 hours to just 20 minutes with comparable quality. The GENSPEED® system is compact, fully automated, and can be used virtually anywhere.

Advantages of the Vitamin D xPOC Test
- Measurement from a drop of blood (50µl) from the fingertip.
- Result after 20 minutes
- Excellent agreement with reference laboratory methods: Pearson correlation coefficient of 0.88 with ELISA and 0.86 with HPLC.
- Informationen about Vitamin D Status
- Measurement range 12 – 100 ng/mL
- Graphical representation with concentration ranges
- The ranges in the legend correspond to the current scientific standards
- Track the individual progression of Vitamin D status over time